You must log in to edit PetroWiki. Help with editing
Content of PetroWiki is intended for personal use only and to supplement, not replace, engineering judgment. SPE disclaims any and all liability for your use of such content. More information
Message: PetroWiki is moving to OnePetro! Please visit PetroWiki's new home here.
Laboratory studies of in-situ combustion
In-situ combustion processes are largely a function of oil composition and rock mineralogy. The extent and nature of the chemical reactions between crude oil and injected air, as well as the heat generated, depend on the oil-matrix system. Laboratory studies, using crude and matrix from a prospective in-situ combustion project, should be performed before designing any field operation.
Reactions
The chemical reactions associated with in-situ combustion are complex and numerous. They occur over a broad temperature range. Most researchers group them into three classes in ascending temperature ranges:
- Low-temperature oxidation (LTO)—heterogeneous gas/liquid reactions producing partially oxygenated compounds and few carbon oxides
- Medium-temperature reactions—cracking and pyrolisis of hydrocarbons to form fuel
- High-temperature oxidation (HTO)—heterogeneous H/C bond breaking reactions in which the fuel reacts with oxygen to form water and carbon oxides
A more recent and more accurate kinetics model has been developed.[1] Only two reactions are used, but in addition, the geometry of the reacting residual fuel in the pore spaces is taken into account, as indicated in Fig. 1. The crude-oil oxidation consists of two stages:
- LTO forming an oxygenated hydrocarbon fuel
- High-temperature combustion of this fuel
Fig. 1 – Schematic diagram of varying fuel geometry.[1]
LTO can be described as oxygen addition to the crude oil. LTO yields water and oxygenated hydrocarbons such as:
- Ketones
- Alcohols
- Peroxides
A good description of LTO can be found in Burger.[2] LTO generally increases the following characteristics of original oil:
- Viscosity
- Boiling range
- Density
LTO increases the amount of fuel and is promoted by low air flux in the oxidation zone. Poor crude oxidation characteristics can also play a role. In heavy oil reservoirs (API gravity < 20°), LTO tends to be more pronounced when oxygen (rather than air) is injected in the reservoir.[3]
A University of Calgary research team has shown that, for heavy oils, LTO reactions must be minimized. Fig. 2 shows the oxygen uptake as the temperature of a typical heavy oil is raised linearly with time. Notice the negative temperature-gradient region, that is, the region in which oxygen-rate uptake decreases with temperature increase. If the temperature of the in-situ combustion process stays at or below the negative temperature-gradient region, the oil displacement efficiency will be very low. This is because LTO increases the oil viscosity and fuel content. The injected-air flux in a heavy-oil project should be maintained at a value well above the value needed to maintain the reactions in the high-temperature oxidation regime. LTO generally has almost no effect on light oils in terms of mobility or recovery despite the fact that light oils are more susceptible to LTO than heavy oils.
Fig. 2 – Schematic of dry combustion temperature profile showing the general effect of temperature on oxygen uptake rate for heavy oils and the negative temperature gradient region.[3]
Fuel deposition determines the feasibility and economic success of a combustion project. It occurs at intermediate temperatures after the LTO reactions. Numerous studies have been conducted in an attempt to understand fuel formation and deposition at intermediate temperatures. The oil type and chemical structure determine the rate and extent of the different reactions. Catalytic effects from the matrix and/or injected solutions of metals may affect the type and amount of fuel formed. Again, all laboratory experiments must include not only the crude to be tested but also representative core material from the reservoir of interest.
Kinetics
Kinetics of combustion reactions can be defined by how fast the chemical reactions occur and how much of the oil is affected. It is important to study kinetics for several reasons:
- Characterization of oil reactivity
- Determination of ignition conditions
- Insight on the nature of the fuel and its combustion characteristics
- Use of kinetic parameters as input for possible numerical simulation of the process
Because crude oils contain hundreds of different compounds, it is impossible to accurately represent all the reactions occurring during in-situ combustion. Even if it were possible to detail all the reactions, the use of such information in numerical models would be impossible because of cost and computer limitations. Consequently, we will concentrate on useful simple models describing in-situ combustion reaction kinetics that have been published in the literature. Most studies use the Arrhenius expressions defined as follows. The model assumes a functional dependency on fuel concentration and oxygen partial pressure. It is given by
-
(1)
where
- RC = reaction rate of the crude, kg/m3s
- Cf = concentration of fuel, kg/m3
- = oxygen partial pressure, Pa.
The exponent constants, a and b, are the orders of the reactions with respect to oxygen partial pressure (a) and fuel concentration (b). Data show that a ranges between 0.5 and 1.0, while b is close to 1.0. The reaction rate, K, is the Arrhenius constant, expressed as a function of temperature as follows:
-
(2)
where
- A = Arrhenius rate constant
- E = activation energy, kJ/mol
- T = absolute temperature, °K
- R = 0.00831 universal gas constant, kJ/mol °K
When using literature values, be careful because the parameters in Eqs. 1 and 2 vary depending on the system of units used.
A variety of experimental techniques can be used to determine the kinetics of in-situ combustion reactions. Among those are:
- Differential thermal analysis
- Thermogravimetric analysis
- Accelerating rate calorimetry
- Effluent analysis
The reference list contains several descriptions of various methods and results.
The effluent analysis method, also called the ramped temperature method in Canada, is quantitative and consists of heating a sample of oil and rock while flowing oxygen (for oxidation) or nitrogen (for pyrolisis) through the sample. The kinetic parameters can be calculated from effluent gas evolution with temperature and chemical analysis of post-test cores. Details of the analysis techniques can be found in the references section.[4][1][5]
Combustion-tube studies
Although the kinetic studies can provide useful insight on in-situ combustion reactions, combustion-tube experiments are mandatory to determine the parameters needed to design and implement field projects. These data are used to make predictions of field test performance. As Sarathi[6] points out, "Combustion-tube studies are the necessary first step in the design of an in-situ combustion project."
Combustion tubes aim at representing a small volume of the reservoir. They are usually packed with native reservoir cores or representative samples of matrix material and oil, placed in vertical position to minimize gravity effects and heated to reservoir temperature. Ignition is usually started at the top by electrical heaters, and the combustion front is propagated downward. This allows propagation of a combustion front and the associated chemical reactions at conditions close to those in a reservoir.
Temperature profiles, pressures, gas and liquid injection and production rates, and composition histories at the inlet and outlet are recorded. ISC tube runs are unscaled, and direct correlation of combustion-tube results to the field is not possible. However, as long as the runs are performed with reservoir rock and fluids at reservoir conditions, the reactions of fuel deposition and combustion will be similar in both tube and reservoir.
Tube runs will not provide information on ISC sweep efficiency. They adequately model the chemistry of the process, but not the flow behavior in the reservoir, and only partially model the heat-transfer processes. Flow behavior in the reservoir is affected by gravity override, well spacing, and geometry and reservoir heterogeneities, and tube runs cannot reproduce these phenomena. Heat transfer from the tube to the surroundings can be much higher than reservoir heat losses.
Two different schools of thought exist on this heat-transfer problem. Many experimenters use strip heaters around the tube to lessen the temperature gradient between the tube and the surroundings. This practice can, however, lead to overestimation of water/oil ratios in wet combustion if the strip heaters provide too much energy to the system, as they often do. Information on front cooling by injected water may also be masked by the heaters. As a result, the extent of the steam plateau may not be correct. Most of these types of experiments are bulky and time consuming and require extensive instrumentation.
The other solution is to increase the air flux and minimize heat losses by insulation alone. This may slightly overestimate air requirements and fuel content but is much simpler and easier to operate. As a result, it is widely used. Descriptions of various setups for combustion-tube studies have been provided.[7][1][8][9][10]
The information that can be acquired from tube runs includes:
- Fuel burned
- Air required to burn a unit volume of reservoir
- Atomic H/C ratio of burned fuel
- Excess air and oxygen use
- Air/fuel ratio
- Oil recovery from the swept zone
- Optimization of water/air ratio in wet combustion
- Composition of produced fluids
- Front temperature and stability
This last piece of information is quite important in heavy oils to determine if the process is operating properly in the desired high-temperature regime. If high temperature cannot be achieved in ideal laboratory conditions, it is likely that field results would be worse.
Data analysis
The following is a simple analysis of data from tube runs. It assumes that the combustion occurs at high temperature where the fuel exclusively combines with oxygen to produce water and carbon oxides. The stoichiometric equation[11] is then
-
(3)
where
- n = hydrogen/carbon atomic ratio of fuel
- m = CO2/CO concentration ratio produced.
The other symbols indicate the various components in the chemical-balance equation.
This equation is only an approximation of the process. It neglects:
- LTO reactions
- Oxygen/mineral reactions
- Water/organic fuel reactions
Alternate analysis when some of these reactions are important is detailed in Sarathi[6] based on work from Moore and Mehta.[8] Assuming Eq. 3 to be valid, the apparent H/C ratio, n, can be estimated from the concentration of exhaust gases and the injected oxygen concentration.[11]
-
(4)
where (O2)prod = oxygen concentration produced.
It is prudent to normalize the concentrations by making a balance on the nitrogen, which in these conditions can be considered inert. The basic chemical equation is then
-
(5)
where
- R is the molar ratio of nitrogen to oxygen in the feed gas
- a, b, d, and f are stoichiometric coefficients similar to those in Eq. 3.
The range of the ratio, n, for high-temperature reactions should be from 0.5 to 2. Calculation of an unusually high value of n indicates that LTO is important. In the very early stages of field projects, large n values are often observed because of the solubility of the combustion gases (particularly CO2) in the oil.
Once n and m are known, the amount of air required to burn one unit weight of fuel is found from Eq. 3. The heat generated by burning a unit weight of fuel can be calculated by simple addition of the heat generated by each reaction, as described in the stoichiometric equation (Eq. 3). The calculation of heat produced must take into account the production of carbon monoxide. The following formula[11] can estimate heating values of fuels as a function of n and m.
-
(6)
where Hc = heating value, Btu/lbm fuel. To convert to J/kg, multiply by 2,326.
The air required to burn a given volume of reservoir is, of course, a very important design parameter and one of the keys to the economics of the combustion process. This is calculated directly from the experimental data by dividing the amount of oxygen consumed by the volume swept during the tube run. The mass of fuel burned in a unit volume of reservoir can be calculated from the oxygen consumed by a unit volume and by applying Eq. 3. All other relevant parameters can be estimated.[11][12] It is prudent to perform multiple laboratory tube runs before field implementation.
Nomenclature
| a, b, d, f | = | stoichiometric coefficients similar to those in Eq. 3 |
| A | = | Arrhenius rate constant |
| Cf | = | concentration of fuel, kg/m3 |
| E | = | activation energy, kJ/mol |
| Hc | = | heating value, Btu/lbm fuel |
| K | = | reaction rate |
| m | = | CO2/CO atomic ratio |
| m | = | CO2/CO concentration ratio produced |
| n | = | hydrogen/carbon atomic ratio of fuel |
| (O2)prod | = | oxygen concentration produced |
| = | oxygen partial pressure, Pa | |
| R | = | 0.00831 universal gas constant, kJ/mol °K |
| RC | = | reaction rate of crude, kg/m3s |
| T | = | absolute temperature, °K |
| Ф | = | porosity, fraction |
References
- ↑ 1.0 1.1 1.2 1.3 Mamora, D. et al. 1993. Kinetics of in-situ combustion. Report No. DOE/BC/14600-51 (DE93000152) SUPRI TR 91. Washington, DC: US Dept. of Energy.
- ↑ Burger, J.G. 1972. Chemical Aspects of In-Situ Combustion - Heat of Combustion and Kinetics. SPE Journal 12 (5): 410-422. SPE-3599-PA. http://dx.doi.org/10.2118/3599-PA
- ↑ 3.0 3.1 Moore, R.G. 1993. New Strategies For In Situ Combustion. J Can Pet Technol 32 (10). PETSOC-93-10-01. http://dx.doi.org/10.2118/93-10-01
- ↑ Burger, J., Sourieau, P., and Combarnous, M. 1989. Thermal Methods Of Oil Recovery. Paris, France: Editions Technip.
- ↑ Fassihi, M.R., Brigham, W.E., Ramey, H.J.J. et al. 1984. Reaction Kinetics of In-Situ Combustion: Part 2—Modeling. SPE J. 24 (4): 408–416. SPE-9454-PA. http://dx.doi.org/10.2118/9454-PA
- ↑ 6.0 6.1 Sarathi, P. 1999. In-Situ Combustion Handbook Principles And Practices. Report DOE/PC/91008-0374, OSTI ID 3175.
- ↑ Bousaid, S. 1989. Multiple-Quenched Fireflood Process Boosts Efficiency. J Pet Technol 41 (11): 1202-1209. SPE-16739-PA. http://dx.doi.org/10.2118/16739-PA
- ↑ 8.0 8.1 Moore, R.G. et al. 1995. A comparison of the laboratory in-situ combustion behaviour of Canadian oils. Proc., 6th UNITAR Conference, Houston.
- ↑ Bardon, C. and Gadelle, C. 1977. Essais de laboratoire pour l’etude de la combustion in-situ. Paper presented at the 1977 French Soviet Symposium on Enhanced Oil Recovery, Moscow.
- ↑ Leaute, R.P. and Collyer, C.J. 1984. Laboratory studies of in situ combustion with cold lake crude. Paper 5 presented at the 1984 Annual Conference on Upgrading Technology and Petroleum Recovery, Calgary.
- ↑ 11.0 11.1 11.2 11.3 Dew, J.N. and Martin, W.I. 1965. How to calculate air requirements for in-situ combustion. Petroleum Engineer.
- ↑ Nelson, T.W. and Mc Neil, J.S. 1961. How to engineer an in-situ combustion project. Producer Monthly (May; Oil & Gas J.
Noteworthy papers in OnePetro
Burger, J.G. and Sahuquet, B.C. 1973. Laboratory Research on Wet Combustion. J Pet Technol 25 (10): 1137–146. SPE-4144-PA. http://dx.doi.org/10.2118/4144-PA
Hascakir, B. Kovscek A., 2014. Analysis of In-Situ Combustion Performance in Heterogeneous Media, SPE Heavy Oil Conference-Canada, 10-12 June, Alberta, Canada https://www.onepetro.org/conference-paper/SPE-170008-MS
Kudryavtsev, P., Hascakir, B., Towards Dynamic Control of In-situ Combustion: Effect of Initial Oil and Water Saturations, 2014 SPE Western North America and Rock Mountain Joint Regional Meeting, 16-18 April 2014 Denver, Colorado USA, SPE 169542-MS., https://www.onepetro.org/conference-paper/SPE-169542-MS
Hascakir, B., Ross, C., Castanier, L. Kovscek, A., Fuel Formation and Conversion During In-Situ Combustion of Crude Oil, SPE Journal, Volume 18, Number 6, December 2013, SPE 146867-PA.https://www.onepetro.org/journal-paper/SPE-146867-PA.
Glatz, G., Hascakir, B., Castanier, L., Kovscek, A., Kinetic Cell and Combustion Tube Results for a Central European Crude, SPE Annual Technical Conference and Exhibition (ATCE), 30 October-2 November 2011 in Denver, Colorado, USA, SPE-146089-PP, https://www.onepetro.org/conference-paper/SPE-146089-MS
Hascakir, B., Glatz, G., Castanier, L. Kovscek, A., In-Situ Combustion Dynamics Visualized with X-Ray Computed Tomography, SPE Journal, Volume 16, Number 3, September 2011, SPE 135186-PA., https://www.onepetro.org/journal-paper/SPE-135186-PA
Cinar, M., Hascakir, B., Castanier, L. Kovscek, A., Predictability of Crude Oil In-Situ Combustion by the Isoconversional Kinetic Approach, SPE Journal, Volume 16, Number 3, September 2011, SPE 148088-PA., https://www.onepetro.org/journal-paper/SPE-148088-PA
Belgrave, J. D. M., Moore, R. G., Ursenbach, M. G., Bennion, D. W., A Comprehensive Approach to In-Situ Combustion Modelling, SPE/DOE 20250 presented at the 7th Symposium on Enhanced Oil Recovery, Tulsa, OK, April 22-25, 1990.
Adegbesan K. O., Donnelly, K. K., Moore, R. G., Bennion, D. W.: Low Temperature Oxidation Kinetic Parameters for In-Situ Combustion Numerical Simulation, SPE 12004 presented at the 58th Annual Technical Conference and Exhibition, San Francisco, CA, Oct. 5-8, 1983.
Moore, R. G., Laureshen, C. J., Ursenbach, M. G., Mehta, S. A., and Belgrave, J. D. M., Combustion/Oxidation Behavior of Athabasca Oil Sands Bitumen, SPE Reservoir Evaluation & Engineering, 2 (6), December 1999.
External links
Use this section to provide links to relevant material on websites other than PetroWiki and OnePetro
See also
Predicting behavior of in-situ combustion
Predicting performance of in-situ combustion
Operating practices for in-situ combustion
![Fig. 1 – Schematic diagram of varying fuel geometry.[1]](/w/images/thumb/f/ff/Vol5_Page_1370_Image_0001.png/300px-Vol5_Page_1370_Image_0001.png)
![Fig. 2 – Schematic of dry combustion temperature profile showing the general effect of temperature on oxygen uptake rate for heavy oils and the negative temperature gradient region.[3]](/w/images/thumb/a/a0/Vol5_Page_1371_Image_0001.png/294px-Vol5_Page_1371_Image_0001.png)

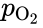 = oxygen partial pressure, Pa.
= oxygen partial pressure, Pa.
![{\displaystyle {\text{CH}}_{n}+\left[\left({\frac {2m+1}{2m+2}}\right)+{\frac {n}{4}}\right]{\text{O}}_{2}=\left({\frac {m}{m+1}}\right){\text{CO}}_{2}+\left({\frac {1}{m+1}}\right){\text{CO}}+\left({\frac {n}{2}}\right){\text{H}}_{2}{\text{O}},}](https://wikimedia.org/api/rest_v1/media/math/render/png/69f59aa4669b9528eac0424f476e55a55975245b)
![{\displaystyle n={\frac {4[{\text{O}}_{2}-{\text{CO}}_{2}-{\text{CO}}/2-({\text{O}}_{2})_{\text{prod}}]}{{\text{CO}}_{2}+{\text{CO}}}},}](https://wikimedia.org/api/rest_v1/media/math/render/png/a9a61d41fd79da39811a0f078b3c9e6a589a9088)

