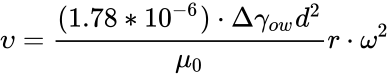You must log in to edit PetroWiki. Help with editing
Content of PetroWiki is intended for personal use only and to supplement, not replace, engineering judgment. SPE disclaims any and all liability for your use of such content. More information
Message: PetroWiki is moving to OnePetro! Please visit PetroWiki's new home here.
Emulsion treating methods
An emulsion treating unit or system will use one or more of the methods listed in Table 1 to aid in destabilizing, coalescence, gravity separation.
Heating
Heating oil emulsions has four basic benefits; It reduces viscosity, increases droplets, dissolves paraffin crystals, and increases density between oil and water.
Viscosity ranges
Crude oil emulsions with similar viscosity ranges do not always require the same type of treating equipment or the same treating temperature. Emulsions that are produced from different wells on the same lease or from the same formation in the same field might require different treating temperatures. For this reason, treating temperatures should be tested so that the lowest practical treating temperature for each emulsion and treating unit or system can be determined by trial.
Free water
The heat input and thus the fuel required for treating depends on the temperature rise, the amount of water in the oil, and the flow rate. Because heating a given volume of water requires approximately twice the energy needed to heat the same volume of oil, it is beneficial to separate free water from the emulsion to be treated. Often this is done in a separate free-water-knockout (FWKO) vessel upstream of where heat is added. Sometimes it is accomplished in a separate section of the same vessel.
Insulated vessel
The required heat input for an insulated vessel (heat loss is assumed to be 10% of heat input) can be approximated using Eq. 1[1][2]:
where
Q = heat input, Btu/hr
ΔT = temperature increase, °F
qo = oil flow rate, B/D
w = water flow rate, B/D
γo = specific gravity of oil
γw = specific gravity of water.
Chemical demulsifiers
Dehydration chemicals, or demulsifiers, are chemical compounds that are widely used to destabilize, and assist in coalescence of, crude-oil emulsions. This treatment method is popular because the chemicals are easily applied, usually are reasonable in cost, and usually minimize the amount of heat and settling time required.
The chemical counteracts the emulsifying agent, allowing the dispersed droplets of the emulsion to coalesce into larger drops and settle out of the matrix. To work, demulsifiers:
- must be injected into the emulsion
- must mix intimately with the emulsion and migrate to all the protective films surrounding all the dispersed droplets
- must displace or nullify the effect of the emulsifying agent at the interface
For the oil and water to separate, there must also be a period of continual, moderate agitation of the treated emulsion to produce contact between and coalescence of the dispersed droplets, as well as a quiet settling period.
One way to help disperse the chemical throughout the emulsion is to mix a small volume of chemical with a diluent and then to inject and mix the diluted chemical with the emulsion. The larger volume of the mixture can help to mix the chemical more uniformly and intimately with the emulsion.
When injection is not recommended
Usually, the chemical is injected into a coupling that is welded in the side of the pipe, but when flow rates are low ( < 3 ft/sec) or when laminar flow is encountered, this is not recommended. In such cases, the following are recommended:
- an injection quill (which injects the chemical in the stream at a location that is removed from the wall)
- a chemical distributor (Fig. 5)
- a static mixer (Fig. 6)
The static mixer is a series of staggered, helically convoluted vanes that use the velocity of the fluid to accomplish mixing.
Fig. 5—Chemical distributor for flowlines 10 in. or larger.[2]
More on chemical demulsifiers see Oil demulsifier selection and optimization.
Wet oil
When a tank of wet oil (oil that contains more than the permissible amount of water) accumulates, the tank contents can be treated by adding a small proportion of demulsifier, agitating or circulating the tank contents, and then allowing time for the water to settle in the tank. Sometimes used for this method of tank treating are railer-mounted units that include:
- Heater
- Circulating pump
- Chemical injector
This batch-treatment method normally is used as an emergency measure.
Chemical measurements
Using too much treating chemical not only wastes the money spent on its purchase, handling, and injection, but also can increase the stability of the water-in-oil emulsion or of the oil-in-water emulsion in the produced water and increase the stability or the volume of the interfacial emulsion and/or sludge. Using too little treating chemical can fail to break the emulsion and can allow a quick buildup of emulsion and/or sludge. It an also:
- cause an excessive need for heat to break the emulsion and for settling time to resolve the emulsion
- reduce the capacity of the treating equipment
- cause high water content in the crude oil and, therefore, the accumulation of unsalable oil and the resultant cost of retreating the crude
- increase the difficulty of removing oil from the produced water
Agitation
Agitation or turbulence is necessary to form a crude-oil emulsion. When turbulence is controlled, however, it can assist in resolving the emulsion. Agitation increases the number of collisions of dispersed particles of water and increases the probability that they will coalesce and settle from the emulsion. Be careful to prevent excessive agitation that will cause further emulsification instead of resolving the emulsion. Keeping the turbulence to moderate Reynolds numbers of 50,000 to 100,000 usually achieves good coalescing conditions.
The flow of emulsions at moderate Reynolds numbers through long pipelines has been shown to cause coalescence and to develop droplets > 1,000 μm in diameter. Using a tortuous flow path as in the serpentine-pipe flow-coalescing device shown in Fig. 7 can decrease the pipeline length required for coalescence. Other devices that are described below have largely supplanted this technology.
Baffle plates
Properly designed and placed baffle plates can assist demulsification by evenly distributing emulsion in a vessel and causing gentle agitation that helps to coalesce the droplets by causing dispersed water particles to collide. Using too much baffling, however, can cause excessive turbulence, which might increase emulsification and impede water-droplet settling. Special perforated baffle plates that are properly placed inside treating vessels provide surfaces on which water droplets can coalesce. The emulsion flowing through the perforations creates slight agitation in the form of eddy currents, which causes coalescence. If the perforations are too small, however, shearing of the water droplets can occur, yielding a tighter emulsion.
Other baffle-plate designs also provide surfaces for water coalescence. The design shown in Fig. 8 allows laminar flow through the plates, but provides directional changes to enable the water droplets to contact the plates and coalesce with a film on the surface of the plates. This type of plate can become plugged if used in situations with high paraffin deposition.
Electrostatic coalescence
The small water drops that are dispersed in the crude oil can be coalesced by subjecting the water-in-oil emulsion to a high-voltage electrical field. When a nonconductive liquid (oil) that contains a dispersed conductive liquid (water) is subjected to an electrostatic field, one of three physical phenomena causes the conductive particles or droplets to combine:
- The water droplets become polarized and tend to align themselves with the lines of electric force. In so doing, the positive and negative poles of the droplets are brought adjacent to each other. Electrical attraction brings the droplets together and causes them to coalesce.
- An induced electric charge attracts the water droplets to an electrode. In a direct current (DC) field, the droplets tend to collect on the electrodes or bounce between the electrodes, forming larger and larger droplets until eventually they settle by gravity.
- The electric field distorts and thus weakens the film of emulsifier surrounding the water droplets. Water droplets dispersed in oil that are subjected to a sinusoidal alternating-current (AC) field become elongated along the lines of force as voltage rises during the first half-cycle. As the droplets are relaxed during the low-voltage part of the cycle, the surface tension pulls them back toward a spherical shape. This effect repeats with each cycle, weakening the film so that it breaks more easily when droplets collide.
Whatever the actual mechanism, the electrical field causes the droplets to move about rapidly, which increases the probability of collision with other droplets. Droplets coalesce when they collide at the proper velocity. The greater the voltage gradient, the greater the forces that cause coalescence; however, experimental data have shown that at some voltage gradient, rather than coalescing, the water droplets can be pulled apart, tightening the emulsion. For this reason, electrostatic treaters normally are equipped with a mechanism for adjusting the voltage gradient in the field.
In oil that contains a large quantity of water, there is a tendency toward “chaining”—the formation of a chain of charged water particles—which might form links between the two electrodes, causing short-circuiting. Chaining has been observed in emulsions that contain 4% or less water. If chaining causes excess power consumption, the voltage gradient is too large (i.e., the electrical grids of the electrostatic treater are too close together or the voltage is too high) for the amount of water being handled. The breaking out of solution of small amounts of gas also can create sufficient turbulence to impede sedimentation.
Water washing
In some emulsion-treating vessels, separation of liquids and vapors takes place in the inlet diverter, flume, or gas boot that is located at the top of the vessel. The liquids flow by gravity through a large conduit to the bottom of the vessel. A spreader plate on the lower end of the conduit spreads the emulsion into many rivulets that move upward through the water, accomplishing a water wash. After passing through the water wash, the emulsion flows to the upper portion of the vessel, where the coalesced water droplets settle out of the oil.
When an emulsion is flowed through an excess of its internal phase, the droplets of its internal phase tend to coalesce with the excess of the internal phase and thus be removed from the continuous phase. This is the principle on which a water wash operates. The water wash is more beneficial if the emulsion has been destabilized by a demulsifier and if the water is heated. The effectiveness of a water wash greatly depends on the ability of the spreader plate or distributor to divide the emulsion into rivulets, causing the emulsion to have maximum intimate contact with the water bath, so that its small drops of water can coalesce with the bulk water.
There is some danger of multiphase emulsion formation if the stream passes through an interface “rag” (unresolved emulsion and solids) layer.
If an emulsion-treating system or unit uses a water wash, it should be charged with water to facilitate initial operation. Water from the emulsion to be treated should be used if available; if not, extraneous water may be used.
Filtering
A filtering material with the proper pore-space size and the proper ratio of pore space to total area can be used to filter out the dispersed water droplets of a crude-oil emulsion by preferentially wetting the filtering material with oil and keeping it submerged in oil. A pack used in this manner is correctly called a filter because it filters out the liquid that it prevents from passing through. Filtering is not a widely used crude-oil-emulsion treatment method, however, because of the difficulty in obtaining and maintaining the desired filtering effect and because filtering materials easily become plugged by foreign material.
One filtering material, excelsior, is wood that has been cut into small shreds or fibers (and so frequently is referred to as “hay”). It formerly was used as a filter in emulsion treaters, but now is largely obsolete. Excelsior should be used at < 180°F treating temperature. Higher temperatures will delignify and deteriorate the excelsior and make it difficult to remove from the vessel.
When its fibers are properly sized and compacted, glass wool also can serve as a filtering material. Coating the glass wool with silicone enhances its filtering effect because the silicone-coated glass-wool fibers are more wettable by oil than are untreated ones. Glass wool is not widely used for filtering, however, because of its initial expense and its fouling problems. Likewise, other available plastic and metal porous filtering materials are not widely used because of the difficulty of obtaining and maintaining the proper pore size and because they easily become inoperable because of fouling.
Fibrous packing
Fibrous coalescing packs are not commonly used in oil treating, but are discussed here for completeness and to differentiate between filtering and coalescence. A coalescing pack is a section or compartment in an emulsion-treating tank or vessel that is packed with a water-wetted material, causing the water in the emulsion to coalesce into larger drops. A coalescing pack works on the principle that two immiscible liquids with different surface tensions cannot simultaneously take possession of a given surface. When the dispersed droplets of water contact the water-wet coalescing material, they coalesce and adhere to the coalescing surfaces. Oil will pass through the pore spaces of the coalescing material. Separation of the two liquids in a coalescing pack, then, is caused not by filtering, but by the greater affinity of the water-wet coalescing material for the water droplets.
The film of oil that contains the emulsifying agent that surrounds the dispersed water particles must be broken before these droplets will adhere to a coalescing medium. This is done with demulsifying chemicals and/or heat and by repeated contact between the water particles and the surface of the coalescing materials while the emulsion flows through the pack. When this film has been broken, the water particles will adhere to the surface of the coalescing material until they combine into drops that are large enough to settle out of the oil.
Glass wool can be used as coalescing material in emulsion-treating vessels, but it fouls easily and might cause channeling. Woven-wire mesh also can be used, but tends to be more expensive than glass wool.
Gravity settling
Gravity settling is the oldest, simplest, and most widely used method for treating crude-oil emulsions. The density difference between the oil and the water causes the water to settle through and out of the oil by gravity. The gravitational force is resisted by a drag force from their downward movement through the oil. When these two forces are equal, a constant velocity is reached that can be computed from Stokes’ law, Eq. 2[1]:
where
v = the downward velocity of the water droplet relative to the oil, ft/sec
d = the diameter of the water droplet, μm
Δγow = the specific-gravity difference between the water and the oil (water/oil)
μo = dynamic viscosity of the oil, cp
Several conclusions can be drawn from Eq. 2:
- The larger the water droplet is, the greater is its downward velocity (i.e., the larger the droplet, the less time it takes to settle to the bottom of the vessel, and thus the easier it is to treat the oil).
- The greater the density difference between the water droplet and the oil, the greater is the downward velocity (i.e., the lighter the oil, the easier it is to treat the oil). For example, if the oil gravity is 10°API and the water is fresh, the settling velocity will be zero because there is no gravity difference.
- The higher the temperature, the lower the oil viscosity, and thus the greater the downward velocity of the water droplets. It is easier to treat the oil at high temperatures than at low temperatures (assuming a small effect on gravity difference because of increased temperature).
Gravity settling can be used alone only to treat loose, unstable emulsions; however, for stronger emulsions, gravity settling separates water from oil only when used with other treating methods that increase water droplet size by destabilizing the emulsion and creating coalescence.
Retention time
In a gravity settler (e.g., an oil-treating tank or the coalescing section of an oil-treating vessel), coalescence will occur, but because of the small forces at work, the rate of contact between water droplets is low, and colliding droplets seldom coalesce immediately. Thus, the coalescence process occurs over time, but it follows a steep exponential curve in which successive doubling of retention time yields small, incremental increases in droplet size.
Adding retention time alone (beyond a small amount for initial coalescence) might not significantly affect the size of the water droplets that must be separated by gravity to meet the desired oil quality. Using a taller tank increases the retention time, but does not decrease the upward velocity of the oil or might not significantly increase the size of the water drops. Thus, the additional retention time gained by using the taller tank might not materially affect the water content of the outlet oil.
Using a larger-diameter tank also will increase the retention time and, more importantly, will slow the upward velocity of the oil, allowing smaller droplets of water to settle out by gravity. In this case, it might not be the increase in retention time that improves the oil quality, but rather the reduction in flow velocity, which decreases the size of the water droplets that can be separated from the oil by gravity.
Centrifugation
Because of the density difference between oil and water, centrifugal force can be used to break an emulsion and separate it into oil and water. Small centrifuges are used to determine the basic sediment and water (BS&W) content of crude-oil emulsion samples. A few centrifuges have been installed in oil fields to process emulsions, but centrifuges have not been widely used for treating emulsions because of:
- high initial cost
- hohe Betriebs- und Wartungskosten
- low capacity
- tendency for fouling
Centrifugal separators are centrifuges for liquid-liquid separation, for liquid-liquid-solid separation or for liquid-solid separation. The basic principle of emulsion separation by means of centrifuges is that described under Eq.2 Stoke's Law . However, disc-stack centrifuges achieve up to 15,000 times the gravitational acceleration (g-force) equivalent to r • ω² . Thus, Stokes Law can be multiplied by the multiples of the g- force.
This means that the sedimentation velocity of a liquid droplet or a in a continuous phase within the disc-stack centrifuge is also multiplied by the same value (g-factor). This allows emulsions and suspensions with a very small droplet / particle size distribution to be separated mechanically into the corresponding phases. Thus the efficiency compared to a static system, which would only separate at 1 x g is significantly higher. If the above-mentioned processes such as chemical demulsification and temperature increase are combined with centrifugation, stabilized emulsions can also be separated into the individual phases with very good efficiency. Centrifugation is gaining in importance due to, for example, the increasing exploitation of heavy oils from matured oil fields, its use in the purification of waste oils and its use in unconventional oil production. Furthermore, disc-stack centrifuges simultaneously separate particles down to a particle diameter of <0.5µm and discharge it independently continuously or periodically from the system. The biggest disc stack centrifuges for such processes can handle feed rates of up to 250 m³/h. For higher feed capacities centrifuges are installed in parallel.
Distillation
Distillation can be used to remove water from crude-oil emulsions. Along with lighter oil fractions, the water can be distilled by heating and then separated by appropriate means. The lighter oil fractions usually are returned to the crude oil.
The only current use of distillation is in the “flash system” that is used in 15°API and lower oil. Flash systems use the excess heat in the oil that is received from the treater or treating system and convert it to latent heat at or near atmospheric pressure. A surface condenser condenses the flashed steam in the cooler, incoming stream of raw crude, thus scavenging the excess heat that ordinarily would be wasted.
Fig. 8 shows a typical flash-distillation system for dehydrating emulsions of heavy viscous crude oils. It is very important in a flash system that the operating pressure be maintained high enough to keep the boiling temperature of the water in the emulsion at least 40°F above the bulk temperature. This will help prevent scale deposition on the heating elements.
The disadvantages of distillation are that it is expensive and that all the dissolved and suspended solids in the water remain in the oil when the water is removed by evaporation. For these reasons, flash treating systems usually are limited to heavy crudes that must meet low BS&W pipeline specifications, as might be the case in cold climates.
Nomenclature
| Q | = | heat input, Btu/hr |
| ΔT | = | temperature increase, °F |
| qo | = | oil flow rate, B/D |
| w | = | water flow rate, B/D |
| γo | = | specific gravity of oil |
| γw | = | specific gravity of water |
| v | = | the downward velocity of the water droplet relative to the oil, ft/sec |
| d | = | the diameter of the water droplet, μm |
| Δγow | = | the specific-gravity difference between the water and the oil (water/oil) |
| μo | = | dynamic viscosity of the oil, cp |
References
Noteworthy papers in OnePetro
Use this section to list papers in OnePetro that a reader who wants to learn more should definitely read
External links
Use this section to provide links to relevant material on websites other than PetroWiki and OnePetro
See also
Sampling and analyzing emulsions
Operational considerations of emulsion treating

![Fig. 5—Chemical distributor for flowlines 10 in. or larger.[2]](/w/images/thumb/b/b4/Vol3_Page_072_Image_0001.png/122px-Vol3_Page_072_Image_0001.png)